Properties
Presentation and Properties
Fentanyl is a synthetic opioid (Fig 1). It comes in 2 ml and 10 ml ampoules, each containing a clear solution of 50 μg/ml. Although fentanyl is very lipid soluble, it is mainly ionized at neutral pH, so is soluble in water.
Pharmacodynamics
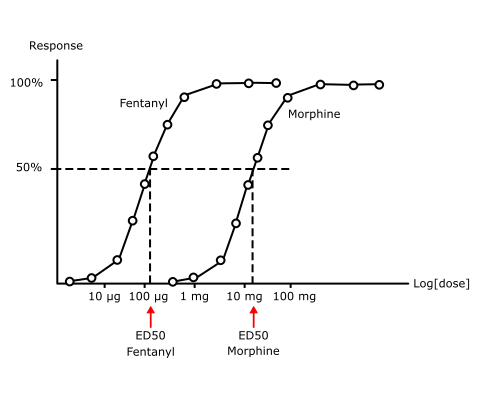
Fentanyl is very potent, about 100 times as potent as morphine (Fig 2). Analgesia is the clinically-relevant effect.
Pharmacokinetics
Table 1 shows the physicochemical properties of fentanyl and morphine. When given as a single intravenous bolus dose, fentanyl is a short-acting drug. Its plasma concentration falls rapidly due to distribution to vessel-rich tissues. If multiple repeated doses or an infusion are used, then there is some accumulation in fat and the recovery time increases. Metabolism, to inactive products, occurs in the liver.
|
Fentanyl |
Morphine | |
|---|---|---|
|
pKa |
8.4 | 8 |
|
Unionized at pH 7.4 (%) |
9 | 23 |
|
Plasma protein bound (%) |
84 | 35 |
|
Relative lipid solubility |
580 | 1 |
|
Terminal half-life (h) |
3.5 | 3 |
|
Clearance (ml/min/kg) |
10-15 | 10-20 |
|
Volume of distribution (L/kg) |
3-5 | 2-3 |
Table 1 Comparison of the physicochemical properties of fentanyl and morphine