Properties
Presentation and Properties
Morphine is a naturally occurring opiate, from the opium poppy (Fig 1). It comes in 1 ml ampoules containing a clear solution of 10 mg/ml. For intravenous use, this is normally diluted with saline to give a solution of 1 mg/ml. Morphine is less lipid-soluble than fentanyl.
Pharmacodynamics
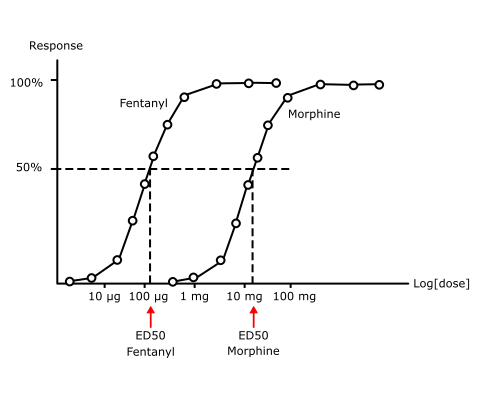
Morphine is less potent than fentanyl (Fig 2). Analgesia is the clinically relevant effect.
Pharmacokinetics
Table 1 shows the physicochemical properties of morphine and fentanyl. Morphine has a slower onset of effect than fentanyl. It is less lipid-soluble, so crosses the blood-brain barrier less rapidly than fentanyl. Morphine has a longer-lasting effect than fentanyl when used as a bolus dose. Its plasma concentration falls relatively rapidly due to distribution to vessel-rich tissues. Metabolism occurs in the liver; an active metabolite is morphine-6-glucuronide, which is more potent than morphine. The presence of an active metabolite is only important in patients with renal failure who receive repeated doses of morphine.
| Fentanyl | Morphine | |
|---|---|---|
|
pKa |
8.4 | 8 |
|
Unionized at PH 7.4 (%) |
9 | 23 |
|
Plasma protein bound (%) |
84 | 35 |
|
Relative lipid solubility |
580 | 1 |
|
Terminal half-life (%) |
3.5 | 3 |
|
Clearance (ml/min/kg) |
10-15 | 10-20 |
|
Volume of distribution (L/kg) |
3-5 | 2-3 |
Table 1 Comparison of the physicochemical properties of fentanyl and morphine